Improving resection rates in borderline resectable pancreatic cancer: Pilot study shows favorable results | The Bulletin

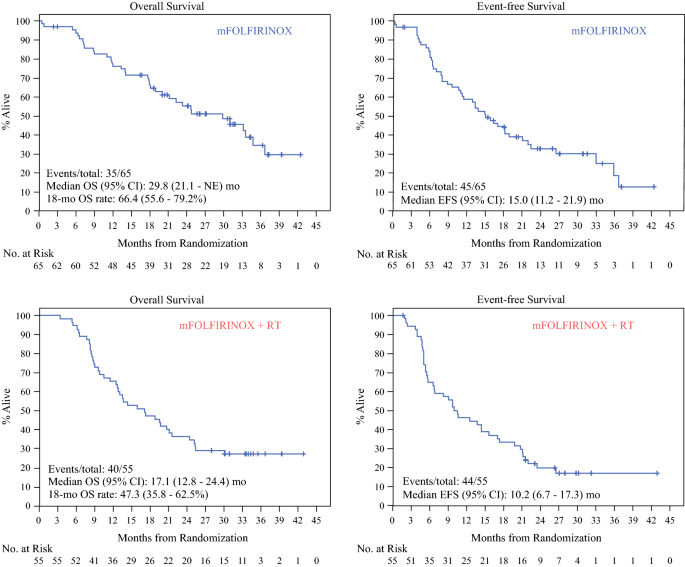
Bassam Estfan on Twitter: "ALLIANCE A021101 of periop mFOLFIRINOX in BRPC. Hard to explain the difference in resectability with the replacement of one cycle by short radiation! Also question choice of short

Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial - The Lancet ...
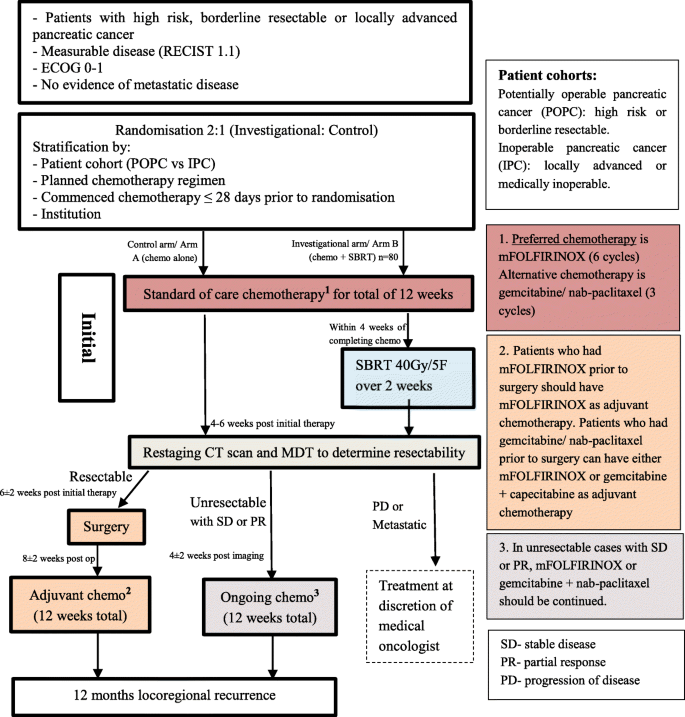
AGITG MASTERPLAN: a randomised phase II study of modified FOLFIRINOX alone or in combination with stereotactic body radiotherapy for patients with high-risk and locally advanced pancreatic cancer | BMC Cancer | Full

Alliance for Clinical Trials in Oncology - Take a look at A021501 – a trial led by @MKatzMD @MDAndersonNews that looks at how well combination chemotherapy and high-dose radiation therapy before surgery

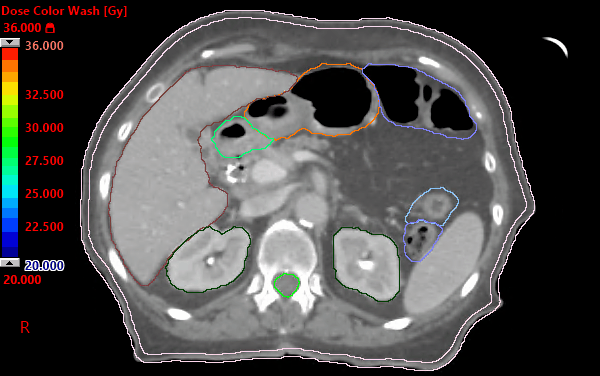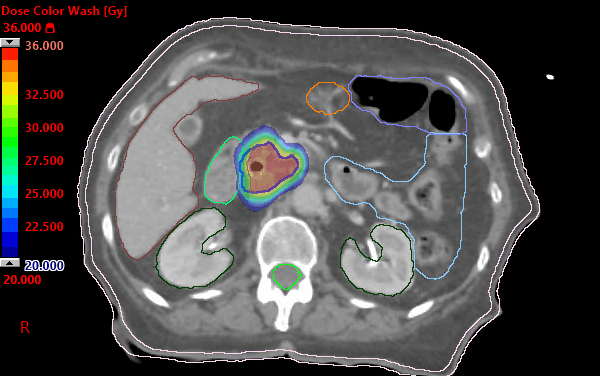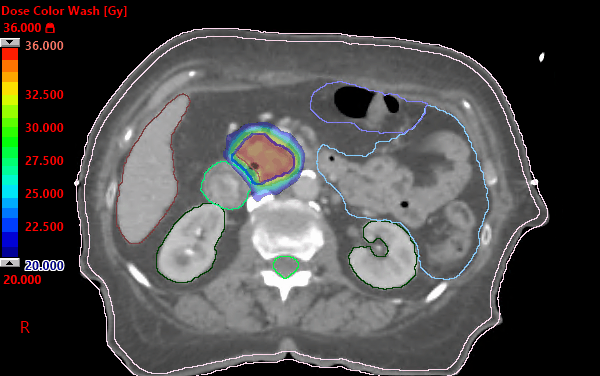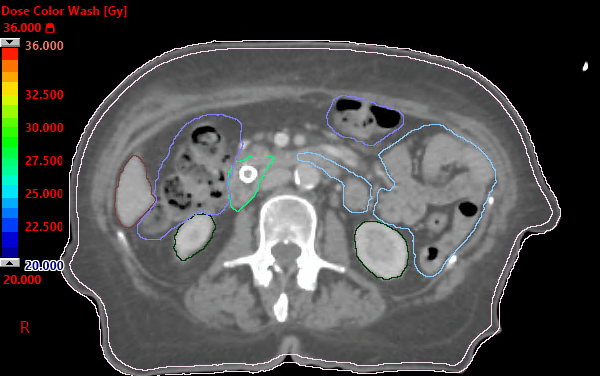
Alliance A021501: preoperative mFOLFIRINOX +/- hypofractionated RT for pancreatic cancer | VJOncology
Stereotactic Versus Conventional Radiation Therapy for Patients With Pancreatic Cancer in the Modern Era

PDF) Alliance for clinical trials in oncology (ALLIANCE) trial A021501: Preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas
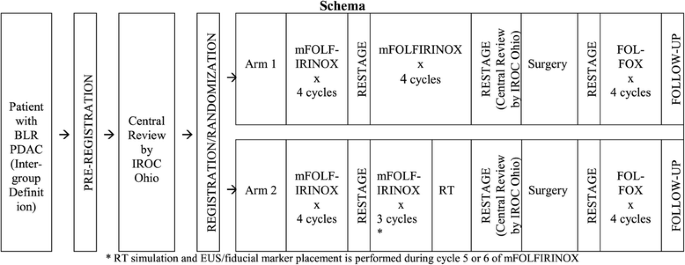
Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas | BMC Cancer

Change in Trial Status: Interim Analysis Started for Alliance Borderline Resectable Pancreatic Cancer Trial – Clancy J. Clark, MD, FACS